A Vision for the Recovery of the Kelp Forests of the Hauraki Gulf
KELSEY MILLER AND NICK SHEARS (University of Auckland)
Kelsey Miller surveying reef at Hauturu after kelp recovery (Credit: Paul Caiger)
The spectacular natural beauty of Te Hauturu-o- Toi/Little Barrier Island is due in part to its stringent terrestrial protections. It became New Zealand’s first nature reserve in 1894 and its pest-free status has led to a healthy abundance of many rare plants and animals today(1). Setting foot on this island is an incredible privilege and requires a permit, but I have been fortunate enough to work in its shallow coastal waters where you can hear its robust chorus of birdsong.
While the terrestrial landscape of the island is a beacon of conservation, the waters surrounding the island tell a very different story. Underwater, the kelp forests that once flourished have receded or are absent, a sign that the marine system is out of balance.
Kelps and fucoids (other large brown seaweeds) frame roughly one fourth of the world’s coastlines, and are among the most productive of all ecosystems(2). They provide habitat, food, and nursery grounds for many fishes, marine mammals, and invertebrates and provide immeasurable ecosystem services(3)(4).
A leading (but not the only) cause of kelp decline is overgrazing by sea urchins(5). When sea urchin populations increase unchecked, they literally eat through forests of kelp, leaving behind urchin barrens (called “kina barrens” in New Zealand). With an absence of kelp and other seaweeds, these areas resemble underwater deserts, with low productivity and a lack of food and habitat for species like crayfish, paua, and kina themselves.
In most parts of the world, the explosion in sea urchin densities is linked to overfishing of their predators, although other factors also play a role in determining the extent and distribution of barrens. In New Zealand, crayfish/kōura (Jasus edwardsii) and large snapper/tāmure (Chrysophrys auratus) are the primary predators of kina Evechinus chloroticus(6). When these predator densities (and individual sizes) become too low, kina densities increase, and kelp disappears in a process known as a trophic cascade. In the Hauraki Gulf, high kina densities and expanses of kina barrens have been attributed to the overfishing of predators for almost 60 years(7). The poor state of crayfish populations in the Hauraki Gulf has been widely demonstrated(8) and they are considered to be ecologically extinct, meaning they no longer play an important role in the ecosystem.
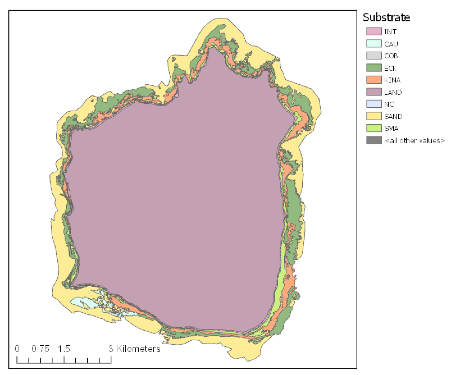
Mapping of Hauturu reef by Dartnall 2022(9)
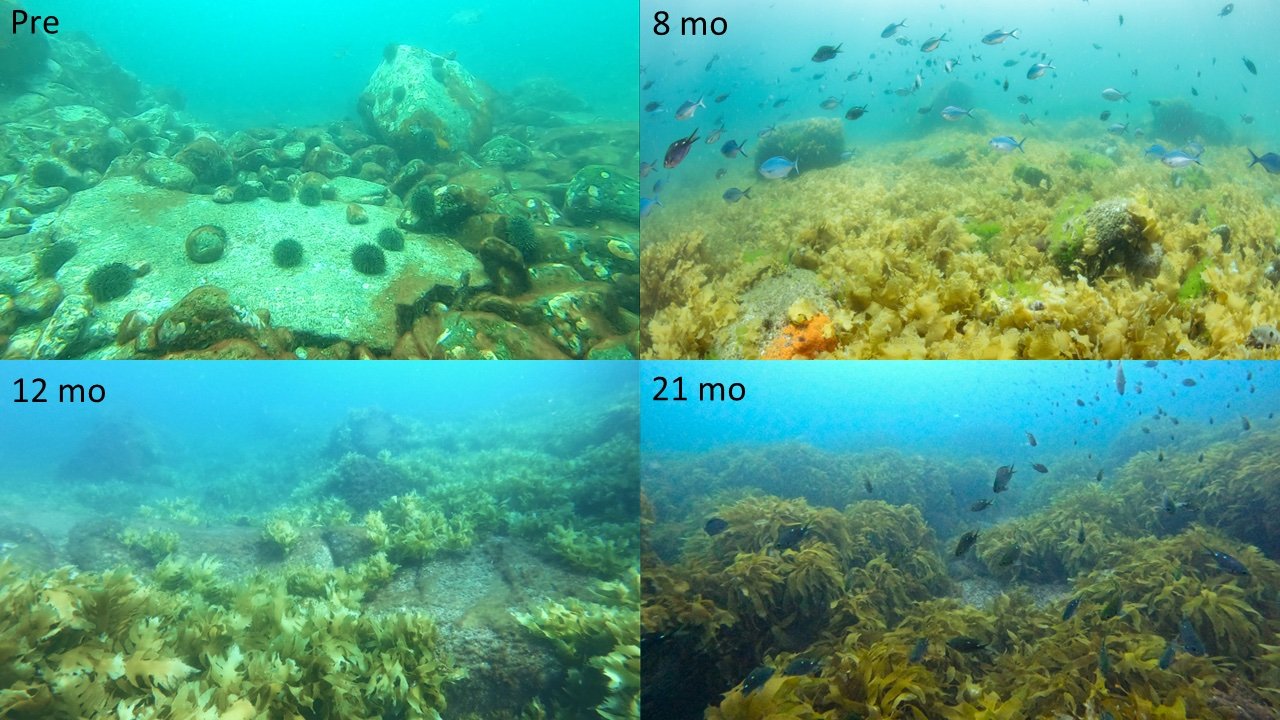
Kelp recovery on Hauturu reef following removal of kina - Removing kina (Credit: Paul Caiger)
In 2021, MSc student Lisa Dartnell mapped the shallow rocky reefs (less than 20 m deep) around Hauturu-o-Toi and found large expanses of kina barren, with an estimated 2.95 square kilometres of barrens(9). Estimating an average of 4 kina/m2 in barrens, this equates to a rough estimate of ~12 million kina in barrens around Hauturu-o-Toi. The earliest published accounts of the reefs at Hauturu-o-Toi demonstrate that the reefs surrounding the island were once lush forests of mixed species of kelp (Ecklonia radiata) and fucoids (primarily Carpophyllum spp. and Sargassum sinclairii). These early records describe a diversity of seaweed in beds interrupted only by patches of sand, and kelp extending from the surface to the depths of visibility – with no mention of kina(10)(11). Aerial imagery from 1953 also show a kelp-dominated reef with an almost complete absence of barrens (0.4%)(9). Now, approximately a third of the underwater forests at Hauturu have been lost to kina barrens.
Through time, coinciding with intensive fishing, kina barrens have emerged in many regions across the country, and particularly in the north-eastern New Zealand and the Hauraki Gulf. Once established, these barrens can be hard to reverse. For example, while it takes a lot of kina to demolish a kelp forest, only a few can eat any new kelp and prevent kelp recovery. To return to a kelp forest state, kina densities must be below roughly 1 kina/square metre(12).
Research from the nearby Cape Rodney-Okakari Point (Te Hāwere-a-Maki, Goat Island) and Tāwharanui marine reserves has shown that with protection of predators, kina barrens will return to kelp forests over time via trophic cascades(13)(14). Snorkelling and diving in these protected kelp forests show us what these reefs can be like: high densities and diversity of fishes, kelps, and invertebrates. Furthermore, within these areas kina still occur in reasonable numbers, but take on a more natural and less destructive habit, living in crevices hidden from predators and feeding on pieces of kelp that drift by.
Here, as in many parts of the world, there is increasing interest in active kelp restoration. Globally, many projects have experimented with sea urchin removal, which normally results in kelp recovery, but is very nuanced15. My PhD research at the University of Auckland with Dr. Nick Shears is evaluating large-scale (1.6-2 ha) kina removal as a tool for kelp restoration - this includes a site at Hauturu-o-Toi. Working closely with Ngāti Manuhiri and other mana whenua, we obtained a special permit from MPI to undertake this research which aimed to understand whether kina removal from barrens promoted kelp recovery and how recovery varied across multiple locations in the Hauraki Gulf. Following kina removal from barrens, we have recorded a remarkable recovery of kelp at all sites in under two years. At Hauturu-o-Toi, kelp cover in the area that was previously kina barrens increased from 7.5% to ~41% over the two-year period. While it is wonderful to see the restoration potential of these undersea forests, especially in such a short time frame, there are many important caveats and considerations in using kina removal for kelp restoration.
Kelp recovery on Hauturu reef following removal of kina - Reef pre-removal (Credit: Sara Kulins), 8 months (Credit: Paul Caiger) 12 months (Credit: Kelsey Miller) and 21 months (Credit: Kelsey Miller) after kina removal.
Active kina removal does not address the cause of kina over-abundance (e.g. overfishing of kina predators), this can only provide partial benefits(15). Kina will come back to the removal areas until we have addressed the issue. In contrast to the flourishing life and lots of fish and crayfish in kelp forests in marine reserves, The vast scale of barrens makes manual removal impractical. It takes more than 50 hours/ha to cull kina in place, and twice that time to collect them all(16). For Hauturu -o-Toi, this equates to roughly 15,000 hours for culling, or 30,000 hours for collecting. Harvest may be an option if the quality of kina in barrens is sufficient (which is typically much lower in barrens), but the fishery is restricted by quotas. While harvest may facilitate kelp recovery and be of value from a kina fishing perspective, it is still not addressing the cause of kina barrens and not restoring a healthy and resilient ecosystem.
Recovered reef at Hauturu (Credit: Paul Caiger)
While many of us who spend time in the water are eager to restore the beloved forests of the sea, we need to be clear on what we hope to accomplish. Knowing how extensive these kelp forests were at Te Hauturu-o-Toi and across the Hauraki Gulf in previous decades, we can see there is much potential for them to flourish again. Our results clearly show that the environmental conditions on reefs in the Gulf can still support productive kelp forests, if kina numbers can be kept under control. While directly removing kina can help to kick-start kelp recovery on small scales, protecting the large predators that once roamed the reefs is needed to provide a long-term approach to restoring the entire reef ecosystem and building resilience. Our work can help to inform protection, restoration, and rebuilding our coastal ecosystems. Increased protection, such as the proposed High Protection Area on the northern side of Hauturu-o-Toi as part of Tai Timu Tai Pari – The Hauraki Gulf Marine Spatial Plan is a start to restore the diverse kelp forest ecosystems and the diversity of life within them that once occurred in this particular area, but is far short of protecting 30% of our marine environment. In addition to large ecosystem-based management approaches, locally led collaborative protection and restoration are also needed for regeneration of our Gulf and beyond.
Restoration options for kina barrens (Image: Miller et al. 2022)
Acknowledgements:
Many thanks to Ngāti Manuhiri and Ngāi Tai ki Tāmaki for their support and partnership through this project. Many thanks to the staff and students at the Leigh Marine Lab and collaborators, without whom this project would not have been possible, in particular: Celia Balemi, Caitlin Blain, Paul Caiger, Sara Kulins, Ohad Peleg, and Arie Spyksma. The research is funded by Foundation North’s Gulf Innovation Fund Together (G.I.F.T) and Live Ocean and Sustainable Seas. Follow us at @nzreefs.
References:
Department of Conservation. (2023). Te Hauturu-o-Toi / Little Barrier Island Nature Reserve. Department of Conservation https://www.doc.govt.nz/parks-and-recreation/places-to-go/auckland/places/little-barrier-island-nature-reserve-hauturu-o-toi/
Mann, K. H. (1973). Seaweeds: their productivity and strategy for growth. Science, 182(4116), 975-981 https://doi. org/10.1126/science.182.4116.975
Christie, H., Norderhaug, K. M., & Fredriksen, S. (2009). Macrophytes as habitat for fauna. Marine Ecology Progress Series, 396, 221-233 https://doi.org/10.3354/meps08351
Graham, M. H. (2004). Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems, 7(4), 341-357 https://doi.org/10.1007/s10021-003-0245-6
Steneck, R. S. (2020). Regular sea urchins as drivers of shallow benthic marine community structure. In Developments in Aquaculture and Fisheries Science (Vol. 43, pp. 255-279). Elsevier https://doi.org/10.1016/b978-0-12-819570-3.00015-9
Babcock, R. C., Kelly, S., Shears, N. T., Walker, J. W., & Willis, T. J. (1999). Changes in community structure in temperate marine reserves [Article]. Marine Ecology Progress Series, 189, 125-134 https://doi.org/10.3354/meps189125
Dromgoole, F. I. (1964, December 1964). Sea-urchins - locusts of the sea. Dive Underwater Magazine, 4(4), 1.
LaScala-Gruenewald, D. E., Grace, R. V., Haggitt, T. R., Hanns, B. J., Kelly, S., MacDiarmid, A., & Shears, N. T. (2021). Small marine reserves do not provide a safeguard against overfishing. Conservation Science and Practice, 3(2), e362 https:// doi.org/https://doi.org/10.1111/csp2.362
Dartnall, L. (2022). The extent of kina barrens over time at Hauturu-o-Toi and the Noises Islands University of Auckland]. Auckland, New Zealand.
Bergquist, P. L. (1960). Notes on the marine algal ecology of some exposed rocky shores of Northland, New Zealand. 1(3- 4), 86-94 https://doi.org/doi:10.1515/botm.1960.1.3-4.86
Trevarthen, C. B. (1953). Features of the marine ecology of Little Barrier, Mayor and Hen Islands. Auckland University Field Club, 6, 34-60.
Shears, N. T., & Babcock, R. C. (2002). Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia, 132(1), 131-142 https://doi.org/10.1007/s00442-002-0920-x
Babcock, R. C., Kelly, S., Shears, N. T., Walker, J. W., & Willis, T. J. (1999). Changes in community structure in temperate marine reserves [Article]. Marine Ecology Progress Series, 189, 125-134 https://doi.org/10.3354/meps189125
Shears, N. T., & Babcock, R. C. (2002). Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia, 132(1), 131-142 https://doi.org/10.1007/s00442-002-0920-x
Miller, K. I., Blain, C. O., & Shears, N. T. (2022). Sea urchin removal as a tool for macroalgal restoration: a review on removing “the spiny enemies” [Systematic Review]. Frontiers in Marine Science, 9 https://doi.org/10.3389/fmars.2022.831001
Miller, K. I., & Shears, N. T. (2023). The efficiency and effectiveness of different sea urchin removal methods for kelp forest restoration. Restoration Ecology, e13754 https://doi.org/10.1111/rec.13754